NHPD Status of Submissions Report
November 2006
The NHPD Status of Submissions Report is a publication of Health Canada's Natural Health Products Directorate (NHPD), the federal department responsible for the regulation of natural health products sold in Canada. Its purpose is to provide the Canadian public with statistical data on the product and site licence applications received and processed by the NHPD.
The NHPD Status of Submissions Report is released to the Canadian public every two months via the NHPD's electronic bulletin. Subscribe to the NHPD e-bulletin.
Questions, comments or feedback regarding the content of the NHPD Status of Submissions Report may be addressed to NHPD_DPSN@hc-sc.gc.ca.
Table of Contents
The NHPD Submission Review Process
Glossary of Terms
Highlight - Significant Progress Made in the Processing of Homeopathic Medicine Product Licence Applications
Product Licensing Statistics
Site Licensing Statistics
Ammendments, Notifications, and Renewals Statistics
The NHPD Submission Review Process
The NHPD submission review process consists of four levels, each of which must be passed before a licence is granted by the NHPD. These are known as Level 1 Processing (where the application is entered into the system and an acknowledgement and submission number is sent); Level 2 Screening (where the application undergoes an initial classification, screening, and is then placed in the assessment queue); Level 3 Assessment (where the application is assessed against the requirements of the Natural Health Products Regulations), and Level 4 Decision (at this stage, following the application's assessment, the decision is made to issue or refuse a licence).

Glossary of Terms
The following is a glossary of terms encountered throughout the report
| Term |
Definition |
| In Queue |
File awaiting assignment to a coordinator/assessor |
| Working |
File has been assigned and is being worked on |
| With Sponsor |
File is awaiting the applicant's response to a PDN or IRN |
| Halted |
File on hold pending an internal decision |
| PC Pending |
File pending a product classification (PC) |
| Refused |
File has been refused by the NHPD due to deficiencies and is no longer being processed |
| Withdrawn |
File removed from the submission review process by the applicant |

Highlight - Significant Progress Made in the Processing of Homeopathic Medicine Product Licence Applications.
Over the past two months, the Natural Health Products Directorate has made significant progress in the processing of product licence applications for homeopathic medicines.
In early September 2006, the total number of homeopathic product licence applications received since January 1, 2004 numbered 654. Of this number, 579 were outstanding (i.e. had not yet been licensed, withdrawn or refused), of which 373 (or 64% of the total outstanding) were sitting in queue at the assessment level (Level 3). However, thanks to a concerted effort between the Directorate's Homeopathic Medicines Unit and Quality Assessment Unit, within a short period of time, the number of applications in queue was cut by over 30% resulting in increases in the number of assessments completed, the number of applications being assessed and the number of applications that have undergone an initial assessment and have been returned to the applicant [removed: ion] for further information.
| |
Early September 2006 |
November 2006 |
| HM applications in queue |
373 |
116 |
| HM applications with the applicant |
26 |
237 |
| HM applications being worked on |
179 |
216 |
| DIN-HMs issued |
7 |
25 |

Product Licensing Statistics
Note - Statistics for this report were generated on November 9, 2006
Chart 1.0 - Flow of Product Licence Applications Received and Processed since January 1, 2006.
Since January 1, 2006, the NHPD has received a total of 5546 new product licence applications and has processed a total of 4452. Since 2006, the average incoming is roughly 1109 new applications every two months while the average outgoing is 890 applications processed every two months.
Please note that "processed" includes the number of licences issued as well as those that were refused by the NHPD and those withdrawn by the applicant.

Chart 2.0 - Flow of Product Licence Applications Processed (i.e. Licensed and Withdrawn/Refused) since January 1, 2006.
Note the significant increases in applications withdrawn and refused from May to September 2006. These were the result of Health Canada's decision to only process complete applications and to refuse those deemed incomplete, which lead to the refusal of several incomplete applications. This course of action was adopted by the Directorate in order to allow it to overcome the current application backlog and expedite the review and assessment of applications.

Chart 2.1 - Product Licence Applications Processed (i.e. Licensed and Withdrawn/Refused) Every Two Months since January 1, 2006.
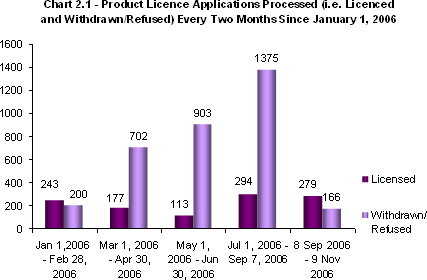
Chart 3.0 - Distribution by Level of Product Licence Applications Currently Being Worked On.
The NHPD is currently working on over 2400 of the total number of product licence applications. These figures also include those applications on hold pending a response from the applicant. They do not include applications that are halted or awaiting a product classification. As Chart 3.0 and the accompanying Table 3.1illustrate, the vast majority of the working files are being actively reviewed in Levels 2 and 3. In addition, over 800 applications are awaiting a response from the applicant.

Table 3.1 - Breakdown Within Each Level of Product Licence Applications Currently Being Worked On
| |
Level 1 |
Level 2 |
Level 3 |
| In Queue |
0 |
65 |
7427 |
| Working |
102 |
774 |
1028 |
| With Sponsor |
28 |
183 |
610 |
| Halted |
0 |
0 |
73 |
| PC Pending |
0 |
57 |
0 |

Site Licensing Statistics
Note - Statistics for this report were generated on November 9, 2006
Chart 4.0 - Flow of Site Licence Applications Received and Processed since January 1, 2006.
Since January 1, 2006, the NHPD has received a total of 200 new site licence applications and has processed a total of 380 applications. Since 2006, the average incoming is roughly 40 new applications every two months while the average outgoing is 76 applications processed every two months.
Please note that "processed" includes the number of licences issued as well as those that were refused by the NHPD and those withdrawn by the applicant.

Chart 5.0 - Flow of Site Licence Applications Processed (i.e. Licensed and Withdrawn/Refused) since January 1, 2006.

Chart 5.1 - Site Licence Applications Processed (i.e. Licensed and Withdrawn/Refused) Every Two Months since January 2006.

Chart 6.0 - Distribution by Level of Site Licence Applications Currently Being Worked On. The NHPD is currently working on 154 of the total number of site licence applications. Over 89% of these are awaiting a response from the applicant.

Table 6.1 - Breakdown Within Each Level of Site Licence Applications Currently Being Worked On.
| |
Level 1 |
Level 2 |
Level 3 |
| In Queue |
0 |
7 |
24 |
| Working |
7 |
4 |
5 |
| With Sponsor |
0 |
73 |
65 |
| Halted |
0 |
0 |
6 |

Ammendments, Notifications, and Renewals Statistics
Amendments and Notifications: Holders of product and/or site licences are required to notify the NHPD of any changes they make to their product or site licence. Depending on the change, the holder must either submit an amendment or a notification.
Renewals: Holders of site licences are required to renew their licences in accordance with a specific renewal schedule.
Further information on amendments, notifications and renewals is available at the following guidance documents:
Note - Statistics for this report were generated on November 9, 2006
Chart 7.0 - Flow of Product Licence Amendments and Notifications Received and Processed since January 1, 2006.

Chart 8.0 - Distribution by Level of Product Licence Amendments and Notifications Currently Being Worked On.

Chart 9.0 - Flow of Site Licence Amendments, Notifications and Renewals Received and Processed since January 1, 2006.

Chart 10.0 - Distribution by Level of Site Licence Amendments, Notifications and Renewals Currently Being Worked On.

|
